May 13, 21 · IL12 inhibitors Pipeline Insights Interleukin12 (IL12) is a heterodimeric protein that consists of IL12, IL23, IL27, and IL35 cytokines, first recovered from EBVtransformed B cell linesJun 01, 14 · To date, some small molecules, such as phosphodiesterase 4 inhibitors, 34 tolllike receptor 4 signaling inhibitor, 35 and 1433ζ modulators, 19 have shown inhibition of IL12/23 production, but none of these compounds have a reported mode of action with the characteristic selectivity observed for APY01Further studies are needed to determine the longterm safety of newer psoriasis treatments (interleukin IL12/23, IL17, Janus kinase 1/3, and phosphodiesterase4 inhibitors), specifically their safety in patients with a history of cancer
Schematic Representation Of Il 12 And Il 23 With Their Receptors And Download Scientific Diagram
List of il-12/23 inhibitors
List of il-12/23 inhibitors-Apr 21, 21 · Clinical data on the use of tocilizumab (and other IL6 inhibitors) for the treatment of COVID19, including data from several randomized trials and large observational studies, are summarized in Table 4b Initial studies that evaluated the use of tocilizumab for the treatment of COVID19 produced conflicting resultsMar 22, 15 · IL23 versus IL12/23 IL23 has been recognized as a major factor in the etiology and pathogenesis of psoriasis, and recent therapeutic development has




Interleukin 12 And Interleukin 23 Blockade In Leukocyte Adhesion Deficiency Type 1 Nejm
May 03, 21 · FDA safety warnings on a pair of JAK inhibitor rivals helped, but with competition mounting, J&J had to slash the price of its IL12/23 inhibitor to stay competitiveAPY01 is a potent and selective IL12/23 inhibitor Products are for laboratory research use only Not for human use We do not sell to patientsHowever, these conventional treatments may not be sufficiently effective and are associated with numerous side effects 2 In recent years, the advent of biological therapy provides new therapeutic options for psoriasis, including interleukin (IL)12/23, IL17, and IL23 inhibitors In particular, IL23 is the latest cytokine discovered to play
Jun 01, 21 · However, these conventional treatments may not be sufficiently effective and are associated with numerous side effects 2 In recent years, the advent of biological therapy provides new therapeutic options for psoriasis, including interleukin (IL)12/23, IL17, and IL23 inhibitors In particular, IL23 is the latest cytokine discovered to playApr 08, · The interleukin (IL)23 inhibitor biologic risankizumab (Skyrizi) showed superior efficacy to placebo in treating moderate to severe plaque psoriasis, according toWhat is the structure of IL12 and 23?
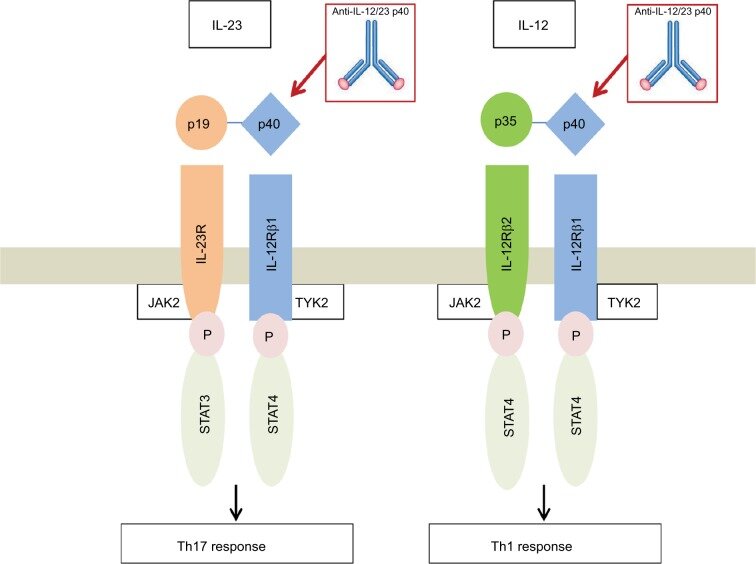
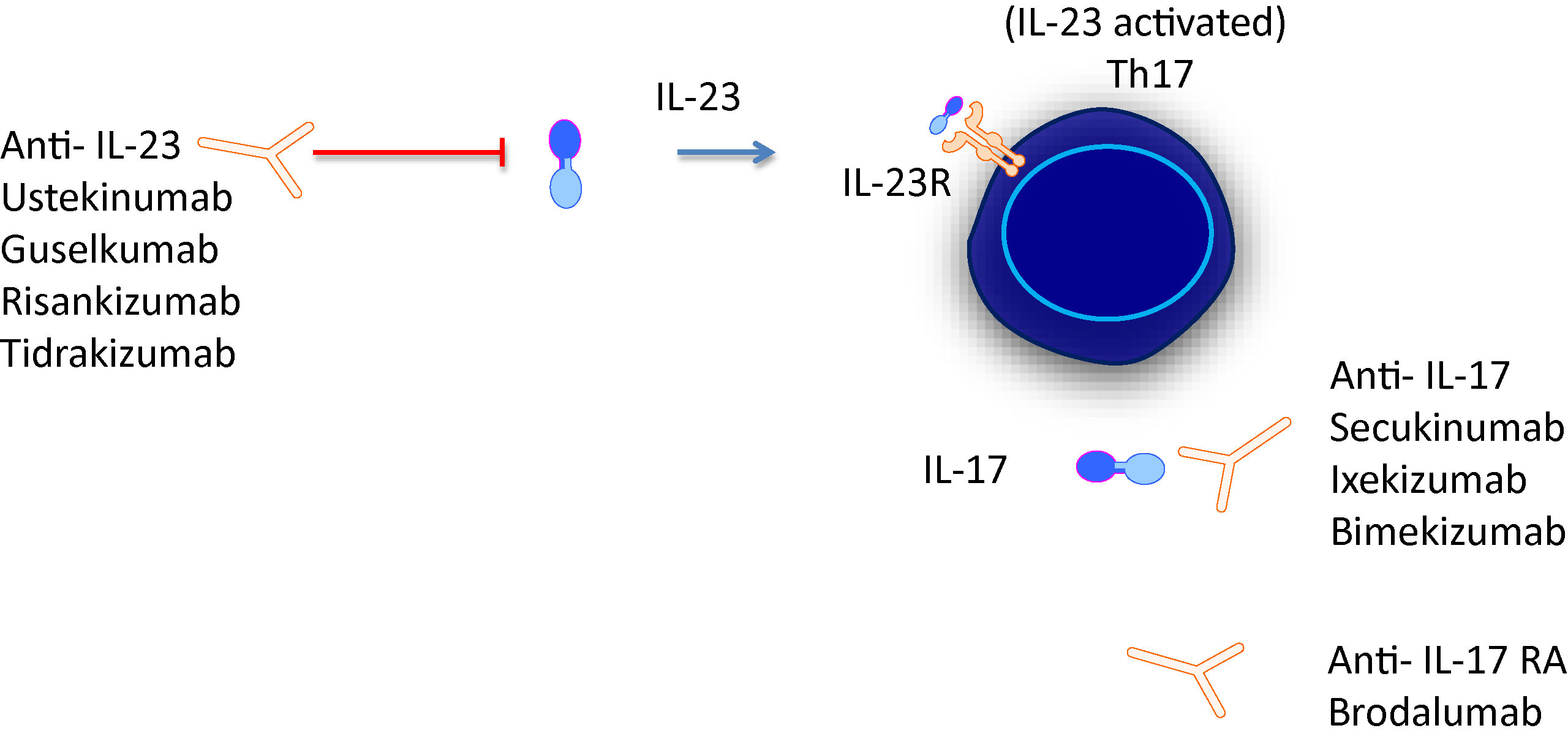
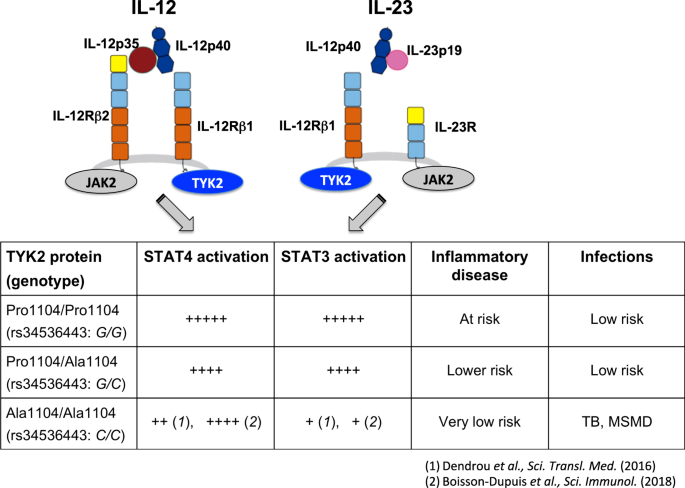
Heterodimer with common p40 subunit that is covalently bound to p35 subunit (bound to IL12) and p19 subunit (IL23) p40 subunit binds the transmembrane IL12 receptor beta 1Mar 23, 21 · The IL23 inhibitors include Stelara (ustekinumab), Tremfya (guselkumab), Ilumya (tildrakizumabasmn) and Skyrizi (risankizumabrzaa) while the IL17 inhibitors include Cosentyx (secukinumab), Taltz (ixekizumab) and Siliq (brodalumab)Apr , 21 · The IL12/23 inhibitor, ustekinumab and the IL23 inhibitors, guselkumab and tildrakizumab, have demonstrated efficacy in psoriasis 3741 Agents that specifically inhibit IL23p19 have demonstrated high efficacy in the treatment of moderatetosevere plaque psoriasis, with a good safety and tolerability profile 36




Inhibition Of Secretion Of Interleukin Il 12 Il 23 Family Cytokines By 4 Trifluoromethyl Celecoxib Is Coupled To Degradation Via The Endoplasmic Reticulum Stress Protein Herp Journal Of Biological Chemistry



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology
The newest biologics for treatment of moderate to severe plaque psoriasis are IL23 and IL17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics Risankizumab, guselkumab, and tildrakizumab are new IL23 inhibitors currently in phase 3 trials with promising early efficacy and safety resultsDrugscom provides accurate and independent information on more than 24,000 prescription drugs, overthecounter medicines and natural products This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment Data sources include IBM Watson Micromedex (updated 3 May 21), Cerner Multum™ (updated 4 May 21), ASHPBS IL12 and 23 are important cytokines that are involved in adaptive immune responses IL23 is important for the development of T helper 17 cells, which are thought to be effector cells in inflammatory bowel disease (IBD) In addition, findings from genetic studies have implicated IL12 and 23 in susceptibility to IBD




Il 23 Inhibitors For Treating Psoriasis What To Know




The Il 23 Axis Genes Associationed With Psoriasis Il 12 And Il 23 Share Download Scientific Diagram
Integrin inhibitors include Entyvio The other good news was that combining therapies, such as taking methotrexate along with a TNF inhibitor, did not have an additional reduction on antibody levels What This Research Means for YouAntiIL23 inhibitors, such as guselkumab, tildrakizumab, risankizumab, orJun 23, 21 · The interleukin (IL)12 and IL23/IL17 axes are implicated as significant pathways in disease pathogenesis5–7 A number of bDMARDs directed against IL12/IL23, IL17 or IL23 are now available to treat PsA, alongside tumour necrosis factor inhibitors (TNFi)8 The IL12/23 axis can be inhibited with ustekinumab, a fully human immunoglobulin



Juan Ovalles Md Phd Ustekinumab An Interleukin 12 23 Inhibitor A Potential New Treatment For Lupus Acr18




Applying Science To Improve The Individualized Treatment Of Patients With Psoriasis Abrar Qureshi Md Mph Chief Of The Department Of Dermatology Rhode Ppt Download
Interleukin23 is a heterodimeric cytokine composed of an IL12B subunit (that is shared with IL12) and the IL23A subunit IL23 is part of IL12 family of cytokines A functional receptor for IL23 (the IL23 receptor) has been identified and is composed of IL12R β1 and IL23R Adnectin2 is binding to IL23 and compete with IL23/IL23R mRNA of IL23R is 2,8 kB in length and includes 12Advances in AntiCytokine Therapy The Current and Potential Role of IL12/23 and IL23 Inhibitors in the Treatment of IBD OnDemand Webcast OVERVIEW The advent of monoclonal antibodies targeting tumor necrosis factor alpha (TNFα) dramatically altered the therapeutic landscape in inflammatory bowel disease (IBD) over the last yearsOct 30, 18 · IL23 inhibitors represent the latest class of therapies to emerge, adding to already available agents, which include TNF inhibitors, IL12/23 inhibitors, and IL17 inhibitors Given the spectrum of potential treatment options available, it is important to understand the role and importance of each class of agent in the therapeutic armamentarium




Il 23 Inhibition In Psoriasis A Novel Approach To Convenient Consistent Clearance European Medical Journal




Short Term Efficacy And Safety Of Il 17 Il 12 23 And Il 23 Inhibitors Brodalumab Secukinumab Ixekizumab Ustekinumab Guselkumab Tildrakizumab And Risankizumab For The Treatment Of Moderate To Severe Plaque Psoriasis A Systematic Review And Network
Ustekinumab or other antiinterleukin12/23 (IL12/23) p40 drugs, antiIL23p19, or antiIL17 or antiIL17 receptor agents in patients with systemic lupus erythematosus Published literature implicates both the IL12 and IL23 pathways in the pathogenesis of systemic lupus erythematosus Ustekinumab is a monoclonal antiIL12 and IL23 antibodyJun 27, 17 · IL12/23 inhibitors Ustekinumab is currently the only approved drug that inhibits the IL12/23p40 subunit, thus antagonizing both IL12 and IL23 110 Ustekinumab is generally considered safe and welltolerated based on both clinical 111, 112 and longitudinal, realworld studies 113 and longterm followup 114Mar 13, · IL23 inhibitors target a type of cytokine called IL23 Cytokines are a class of proteins that help transmit signals from one cell to another IL23 plays a




Inhibition Of Interleukin 12 And Or Interleukin 23 For The Treatment Of Psoriasis What Is The Evidence For An Effect On Malignancy Ergen 18 Experimental Dermatology Wiley Online Library



The Il 23 Il 17 Pathway In Human Chronic Inflammatory Diseases New Insight From Genetics And Targeted Therapies Genes Immunity
The psoriasis therapy market has become increasingly lucrative owing to the growing use of targeted agents The dominance of the tumor necrosis factor (TNF)alpha inhibitors and interleukin (IL)12/23 inhibitor ustekinumab (Janssen's Stelara) has been challenged by novel, highly effective IL17 and IL23 inhibitorsIn particular, the uptake of risankizumab (AbbVie's Skyrizi)—theIntroduction The monoclonal antibody targeting the shared p40 subunit of IL12 and IL23, namely ustekinumab, has been approved for Crohn's disease (CD) and has demonstrated promising results in the treatment of ulcerative colitis Several agents targeting the IL23specific p19 subunit are currently in various stages of developmentApr , 21 · If approved, Skyrizi would be the second selective IL23 inhibitor available for the disease and third IL23 inhibitor overall, following Janssen's IL12/23 inhibitor Stelara (ustekinumab) and selective IL23 inhibitor Tremfya (guselkumab) Although key opinion leaders (KOLs) interviewed by GlobalData have had a generally positive reaction to



Radiation Inhibits Interleukin 12 Production Via Inhibition Of C Rel Through The Interleukin 6 Signal Transducer And Activator Of Transcription 3 Signaling Pathway In Dendritic Cells




Il 23 Inhibitors For Psoriasis Therapeutic Cheat Sheet Next Steps In Dermatology
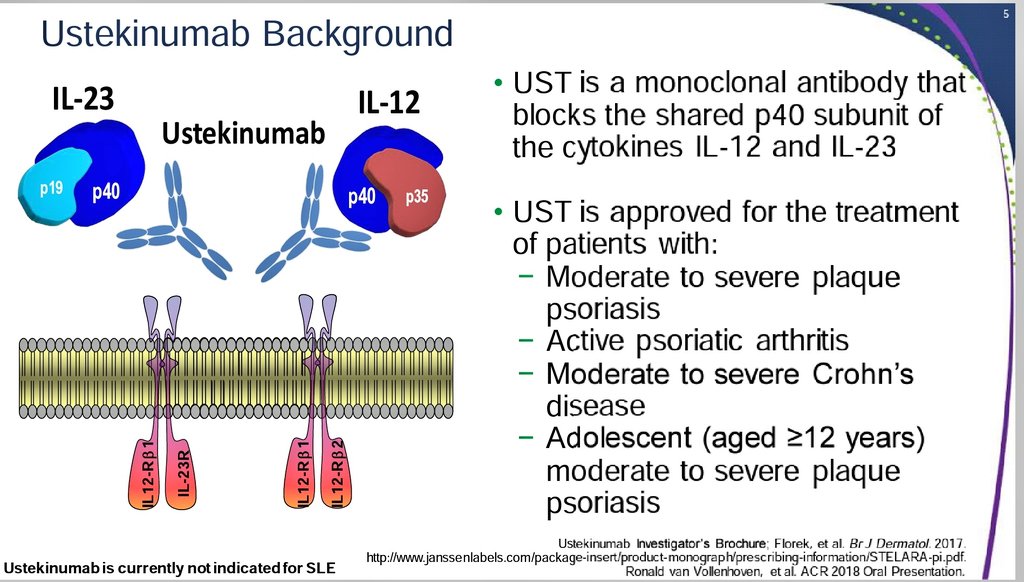
Background/Purpose Both the IL12 and IL23 pathways have been linked to SLE pathogenesis The antiIL12/23 p40 monoclonal antibody ustekinumab (UST), which is approved for psoriasis, PsA, and Crohn's disease, was evaluated in patients with active SLEInterleukin (IL) 12/23 inhibitors are parenteral biologic monoclonal antibodies that bind the p40 subunit shared by IL 12 and IL 23 and include the agents, ustekinumab and guselkumab Ustekinumab is currently the only such agent available and is approved for use in psoriasis, psoriatic arthritis and inflammatory bowel diseaseJun 01, · TNFα inhibitors were the first biologics introduced for PSO treatment and include etanercept, infliximab, adalimumab, and certolizumab, but other new inhibitors are more effective and have better safety profiles, including antiIL12/23 inhibitors such as UTK;




Neutralization Of The Interleukin 12 Interleukin 23 Pathways Associated Download Scientific Diagram




Schematic Representation Of Il 12 And Il 23 With Their Receptors And Download Scientific Diagram
Nov 19, 19 · IL12/23 Inhibitors / IL23 Inhibitors Ustekinumab (Stelara ® ) is a monoclonal antibody that targets IL12 and IL23, whereas guselkumab (Tremfya TM ), risankizumab, and tildrakizumab are monoclonal antibodies that target IL23Psoriasis is a common chronic inflammatory skin disease that is mediated, in part by the body's Tcell inflammatory response mechanisms Further insight into the pathogenesis of the disease and the role of various cytokines, particularly interleukin(IL)12 and IL23, has led to advances in the treatment of this diseaseApr 09, · With more than 81 000 deaths worldwide from coronavirus disease 19 (COVID19) by April 8, ,1 it is incumbent on researchers to accelerate clinical trials of any readily available and potentially acceptably safe therapies that could reduce the rising death toll Severe acute respiratory syndrome coronavirus 2 (SARSCoV2) gains access to host cells via angiotensin
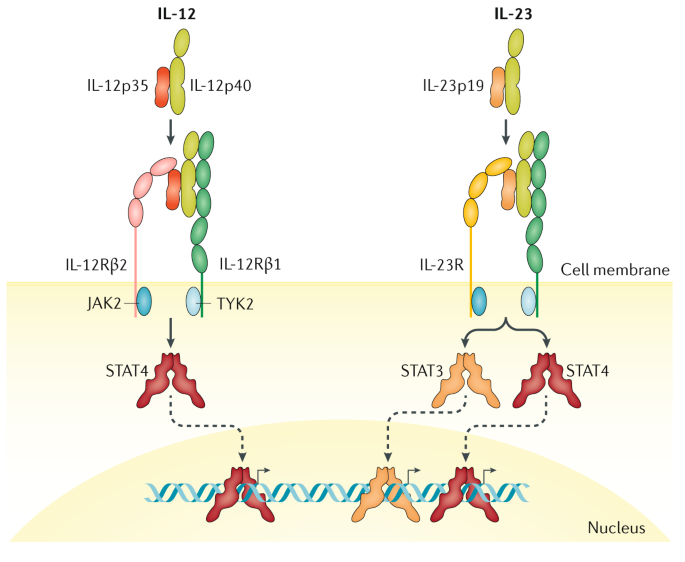



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




The Other Side Of The Moon A Clinical Dialogue On The Il 23 Pathway European Medical Journal
Nov 25, 12 · Although the number of different leukocyte cell types that are known to be able to respond to IL12 and IL23 is growing 12,13, the role of IL12/IL23 signaling in the context of amyloidinducedMar 24, · An IL12/23 inhibitor may be effective when PsA has not responded well to TNF inhibitors or IL17 inhibitors Alternately, a doctor may prescribe an IL12/23 inhibitor if a person has both PsA andMar 05, 21 · Notably, another IL12/23 inhibitor, briakinumab, increased the risk of MACE in 5 studies, resulting in the discontinuation of all briakinumab trials in 11 Tofacitinib, a Janus kinase inhibitor, has been approved for use in psoriasis and is associated with a low incidence of MACE
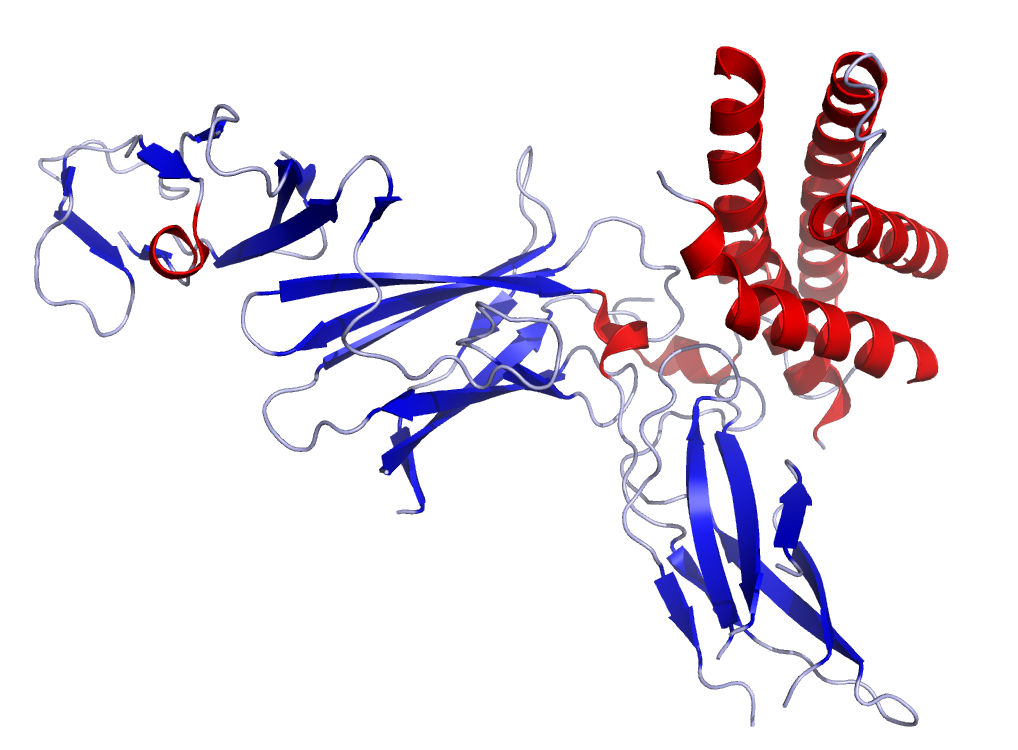



Interleukin 12 Wikipedia




Ustekinumab A Human Interleukin 12 23 Monoclonal Antibody For Psoriatic Arthritis Randomised Double Blind Placebo Controlled Crossover Trial The Lancet
Apr 09, 21 · IL12/23 or IL23 inhibitors include such therapies as Stelara, Tremfya, and Skyrizi;Mar 19, 21 · First, IL17A blockers have proven efficacy for both peripheral and axial SpA including evidence for efficacy for isolated enthesitis as a secondary outcome measure (46–50) Likewise, the published literature shows efficacy for IL12/IL23 p40 blockers for peripheral PsA and for isolated enthesitis (30, 51–53)Jan 21, 21 · The results of several clinical trials have shown novel biologic agents such as ixekizumaband guselkumab to be highly effective for patients with moderate to severe psoriasis 69 Recently approved by the FDA as the first selective IL23 inhibitor for the treatment of psoriatic arthritis, guselkumab is a human immunoglobulin G1λ monoclonal antibody targeting the p19




A Balance Of Interleukin 12 And 23 In Cancer Trends In Immunology



Full Article Monoclonal Antibodies Inhibiting Il 12 23 And 17 For The Treatment Of Psoriasis
Mar 25, 08 · A Randomized, Doubleblind, Placebocontrolled Clinical Study of the Oral IL12/23 Inhibitor, STA5326 Mesylate, Administered to Patients With Rheumatoid Arthritis to Determine Safety, Tolerability, Pharmacokinetic and Synovial Tissue Outcomes Full Text ViewMay 12, · Bilal J, Berlinberg A, Bhattacharjee S, Trost J, Riaz IB, Kurtzman DJB A systematic review and metaanalysis of the efficacy and safety of the interleukin (IL)12/23 and IL17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasisDermatology Times, Dermatology Times, December 18 (Vol 39, No 12), Volume 39, Issue 12 Conference Fall Clinical Dermatology Two approved and two pending antiIL 23 drugs offer an opportunity for moderate to severe psoriasis patients to regain their lives, according to researchers presenting at the 18 Fall Clinical Dermatology Conference
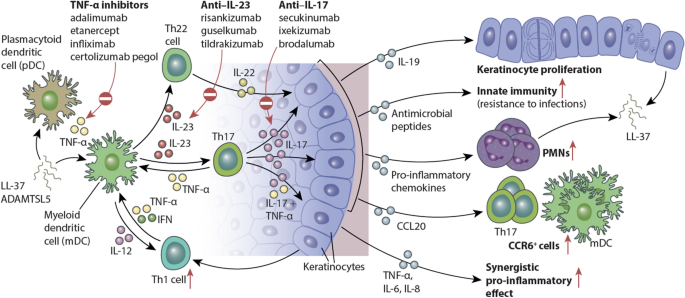



Interleukin 17 And Interleukin 23 A Narrative Review Of Mechanisms Of Action In Psoriasis And Associated Comorbidities Springerlink




Il 23 Antagonists In The Treatment Of Plaque Psoriasis Dermatology Advisor




Interleukin 12 Interleukin 23 And Psoriasis Current Prospects Journal Of The American Academy Of Dermatology
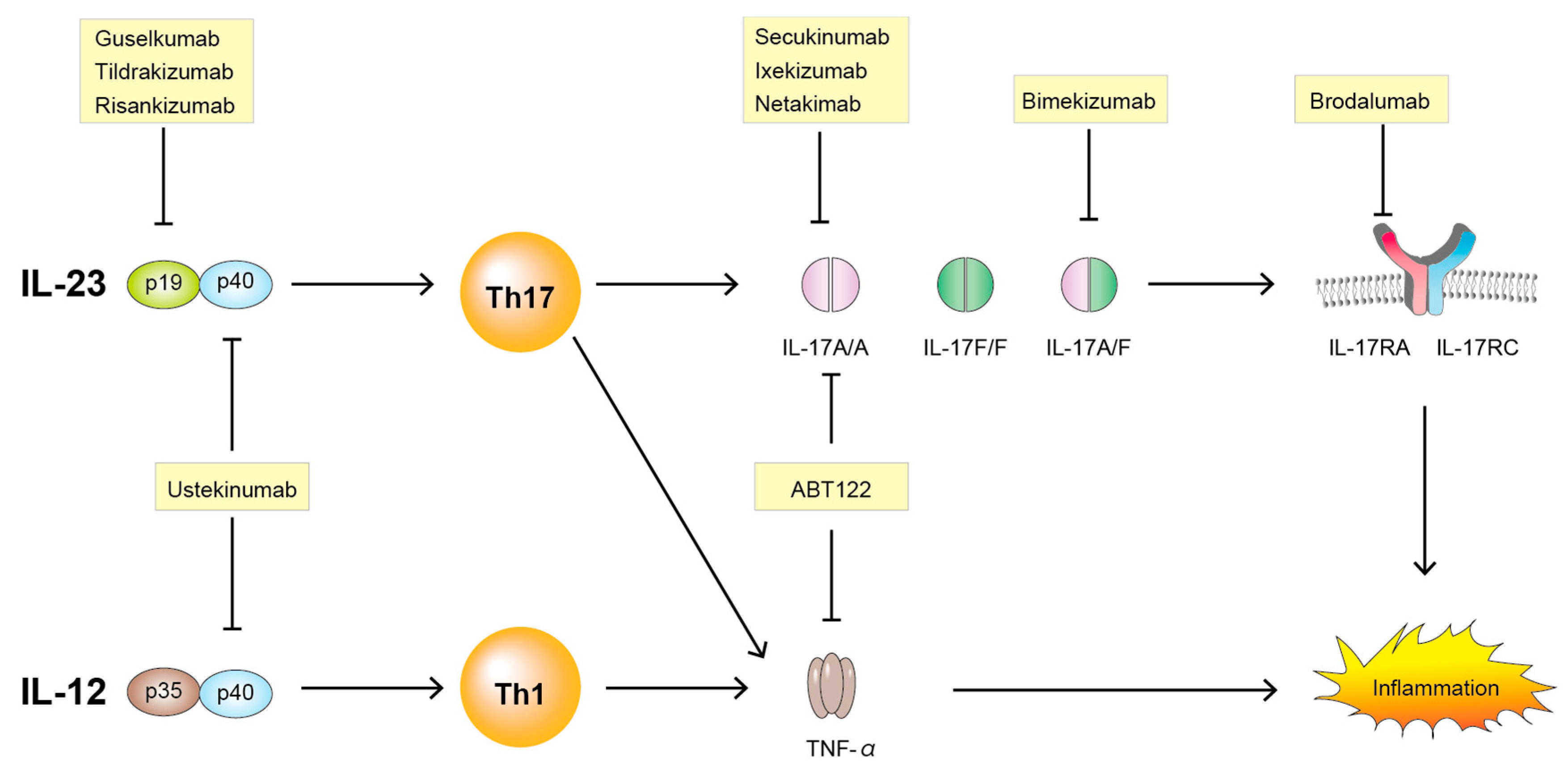



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html




Pdf Short Term Efficacy And Safety Of Il 17 Il 12 23 And Il 23 Inhibitors Brodalumab Secukinumab Ixekizumab Ustekinumab Guselkumab Tildrakizumab And Risankizumab For The Treatment Of Moderate To Severe Plaque Psoriasis A Systematic Review And




Positioning Anti Il 12 And Anti Il 23 Inhibitors Youtube




Structures Of Il 12 And Il 23 Their Receptors And The Site Of Action Download Scientific Diagram




New Biologics And Small Molecules Under Development For The Treatment Download Scientific Diagram




Bio X Cell Invivomab Anti Mouse Il 12 P40




Shifting The Focus The Primary Role Of Il 23 In Psoriasis And Other Inflammatory Disorders Abstract Europe Pmc
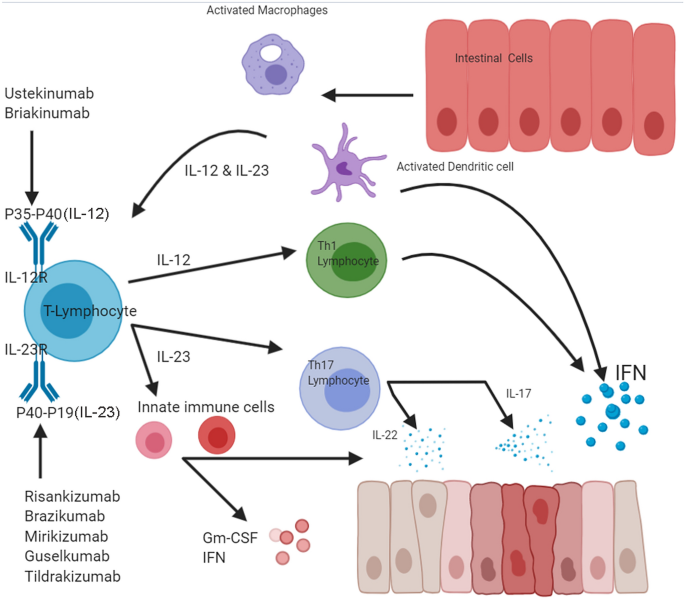



Clinical Trials Of Il 12 Il 23 Inhibitors In Inflammatory Bowel Disease Springerlink
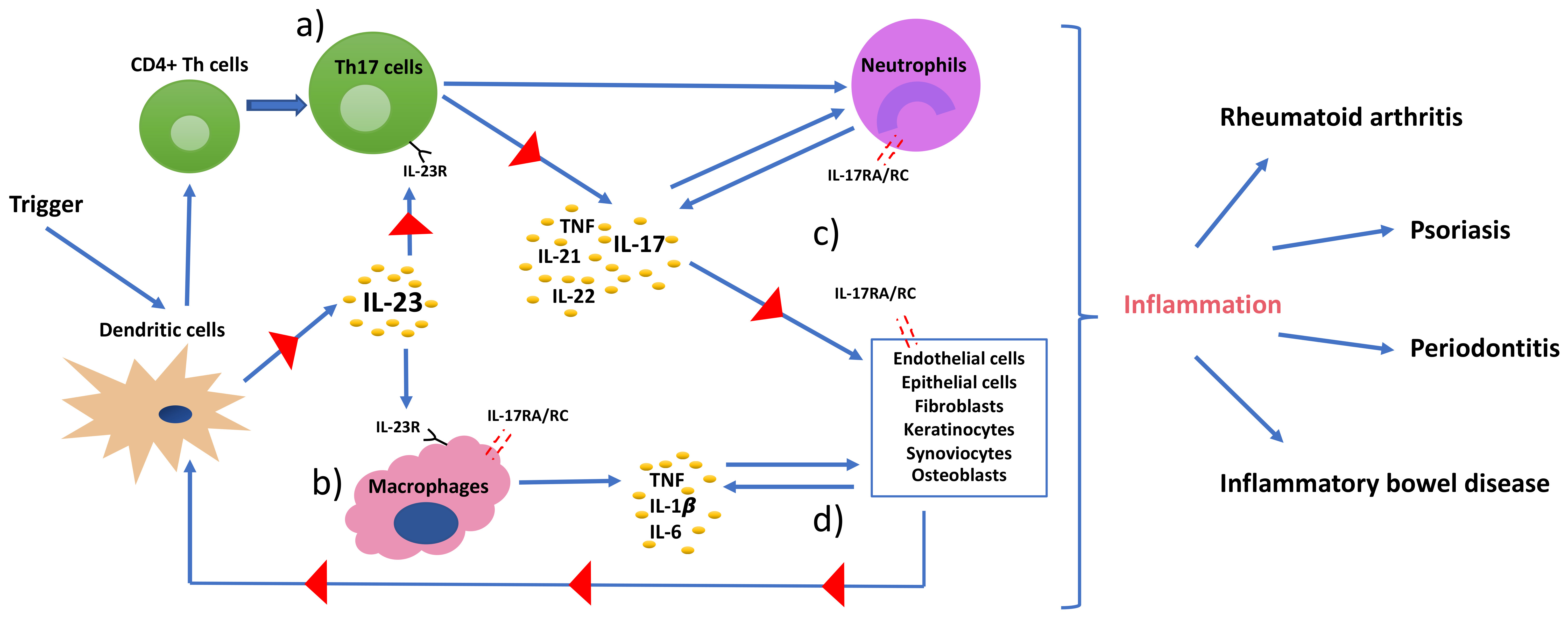



Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html




Il 12 23 Inhibitors And Small Molecule Inhibitors Youtube




A Balance Of Interleukin 12 And 23 In Cancer Trends In Immunology




Heterodimeric Cytokines Belonging To Il 12 Family Structural And Download Scientific Diagram
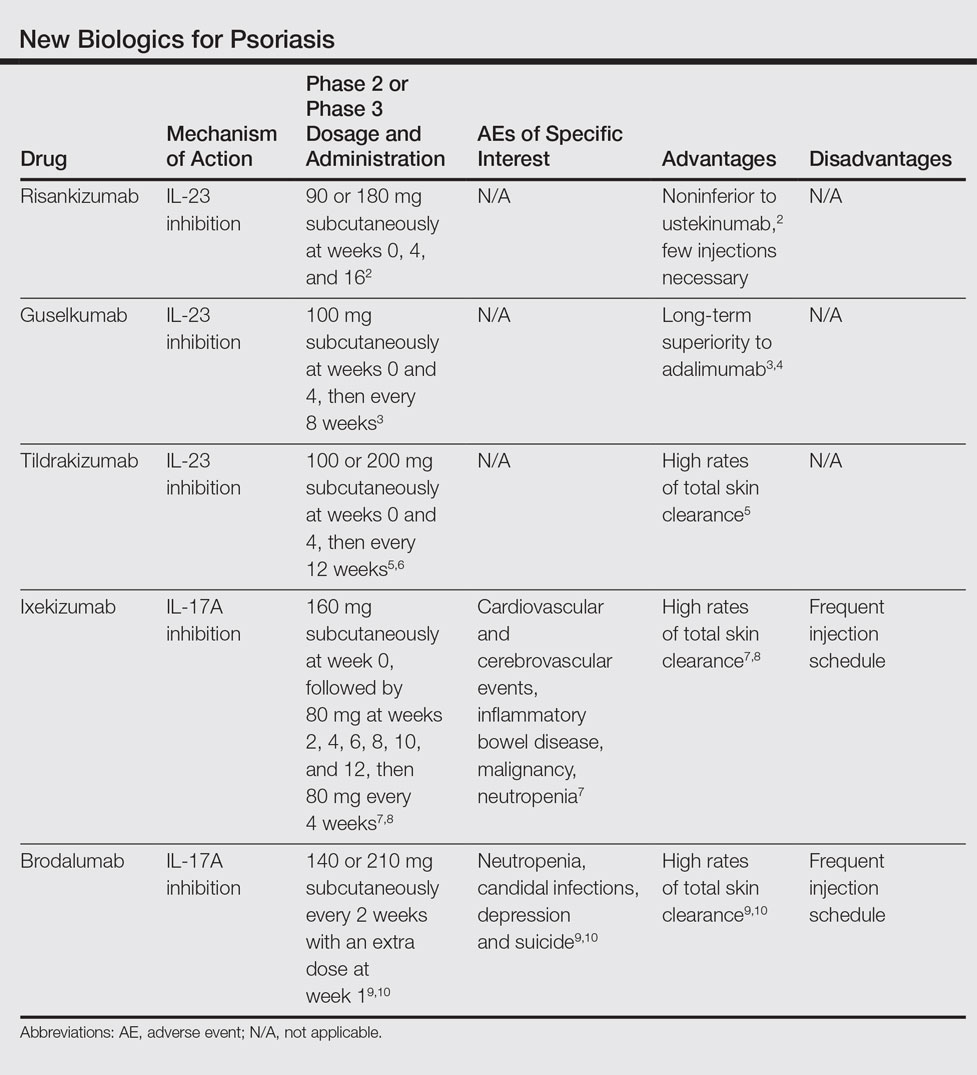



New Biologics In Psoriasis An Update On Il 23 And Il 17 Inhibitors Mdedge Dermatology




Targeting Interleukin 23 In The Treatment Of Noninfectious Uveitis Ophthalmology




Advancing The Treatment Of Psoriatic Arthritis Focus On The Il 23 Pathway European Medical Journal




The Tnf Il 23 Il 17 Axis Head To Head Trials Comparing Different Biologics In Psoriasis Treatment Ten Bergen Scandinavian Journal Of Immunology Wiley Online Library
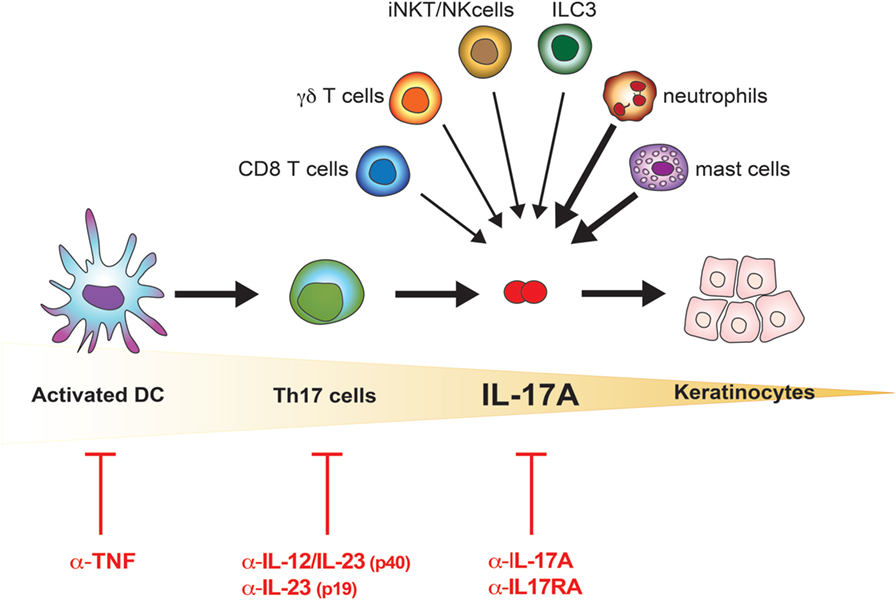



Frontiers The Il 17 Family Of Cytokines In Psoriasis Il 17a And Beyond Immunology




Interleukin 12 23 Deficiency Differentially Affects Pathology In Male And Female Alzheimer S Disease Like Mice Embo Reports




Ustekinumab Binds To The P40 Subunit Of Il 12 And Il 23 Preventing Download Scientific Diagram
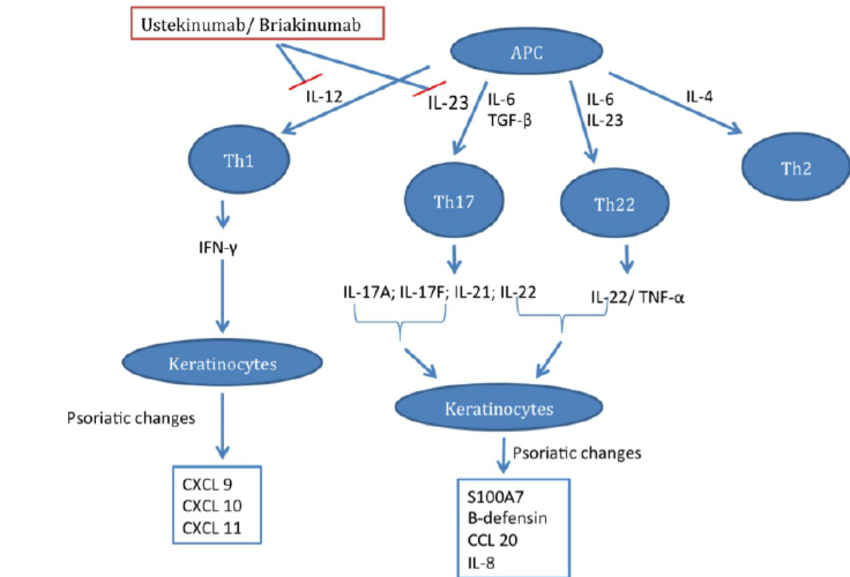



The Pivotal Role Of Some Of Il 12 And Il 23 In Psoriasis Download Scientific Diagram
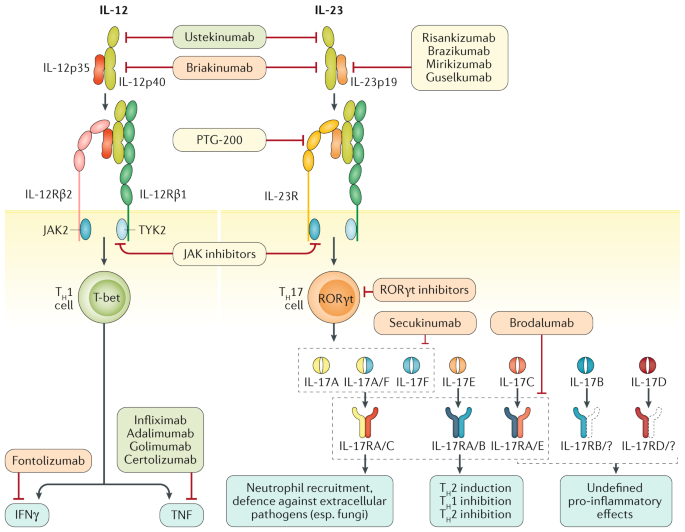



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




The Role Of Il 23 And The Il 23 Th17 Immune Axis In The Pathogenesis And Treatment Of Psoriasis Girolomoni 17 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library
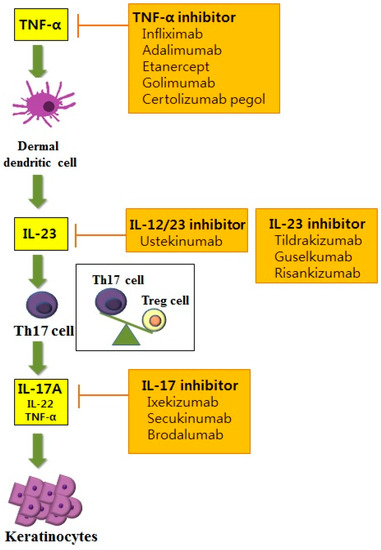



Ijms Free Full Text Molecular Mechanisms And Management Of A Cutaneous Inflammatory Disorder Psoriasis Html




The Il 23 Th17 Axis In The Immunopathogenesis Of Psoriasis Sciencedirect




Interleukin 12 And Interleukin 23 Blockade In Leukocyte Adhesion Deficiency Type 1 Nejm




New Treatments For Atopic Dermatitis Targeting Beyond Il 4 Il 13 Cytokines Annals Of Allergy Asthma Immunology




Cutting Edge Il 23 Cross Regulates Il 12 Production In T Cell Dependent Experimental Colitis The Journal Of Immunology
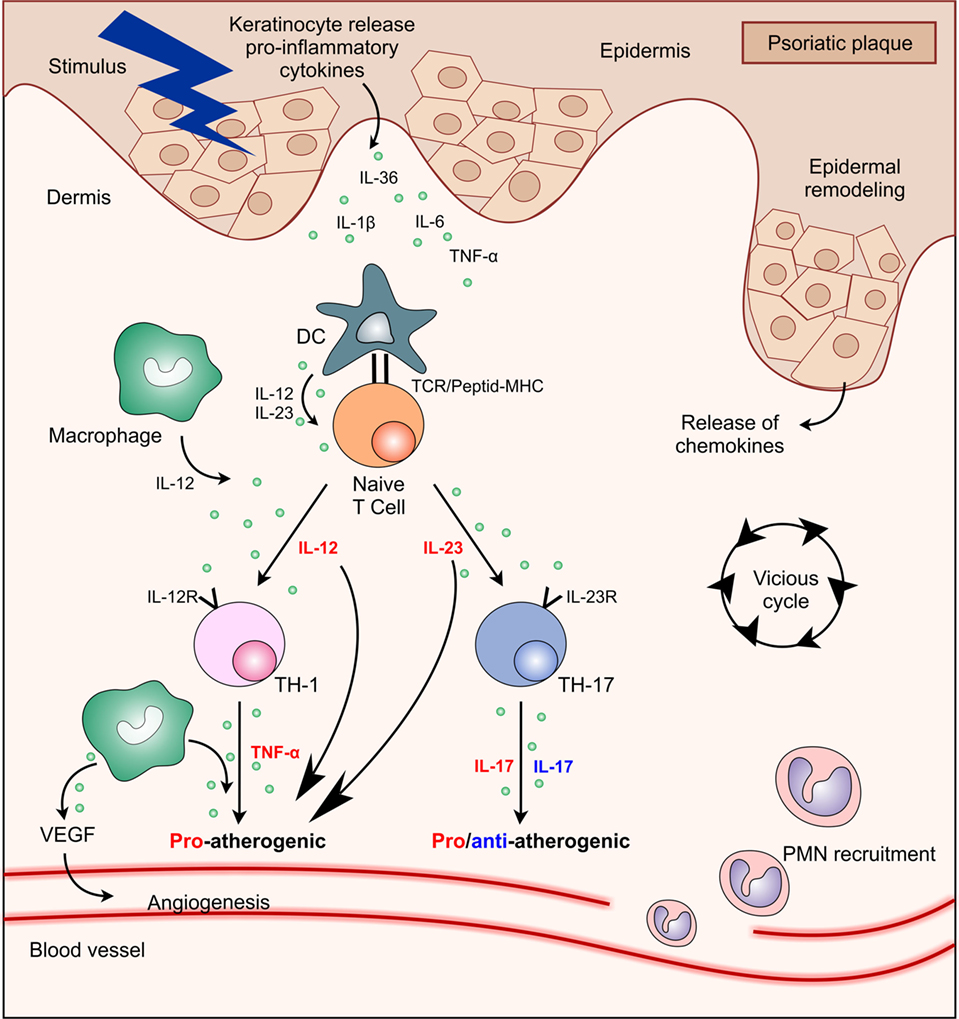



Frontiers Psoriasis Cardiovascular Events And Biologics Lights And Shadows Immunology




Biologic Therapies For Psoriasis Plastic Surgery Key




Pathophysiology And Inhibition Of Il 23 Signaling In Psoriatic Arthritis A Molecular Insight Sciencedirect




Il 12 23 Inhibitors Linked To Lower Infection Risk Than Tnf Il 17 Inhibitors In Psa Psoriasis




Interleukin 23 Inhibition As A Strategy To Treat Immune Mediated Inflammatory Diseases European Medical Journal




Role Of Il 12 Il 23 In The Pathogenesis Of Multiple Sclerosis Sciencedirect




Fine Tuning The Treatment Of Psoriatic Arthritis Focus On The Il 23 Pathway European Medical Journal




Role Of Il 12 Il 23 In The Pathogenesis Of Multiple Sclerosis Sciencedirect




Anti Il 12 23 P40 Antibody Attenuates Chronic Graft Versus Host Disease With Lupus Nephritis Via Inhibiting Tfh Cell In Mice Sciencedirect




The Immunobiology Of The Interleukin 12 Family Room For Discovery Sciencedirect
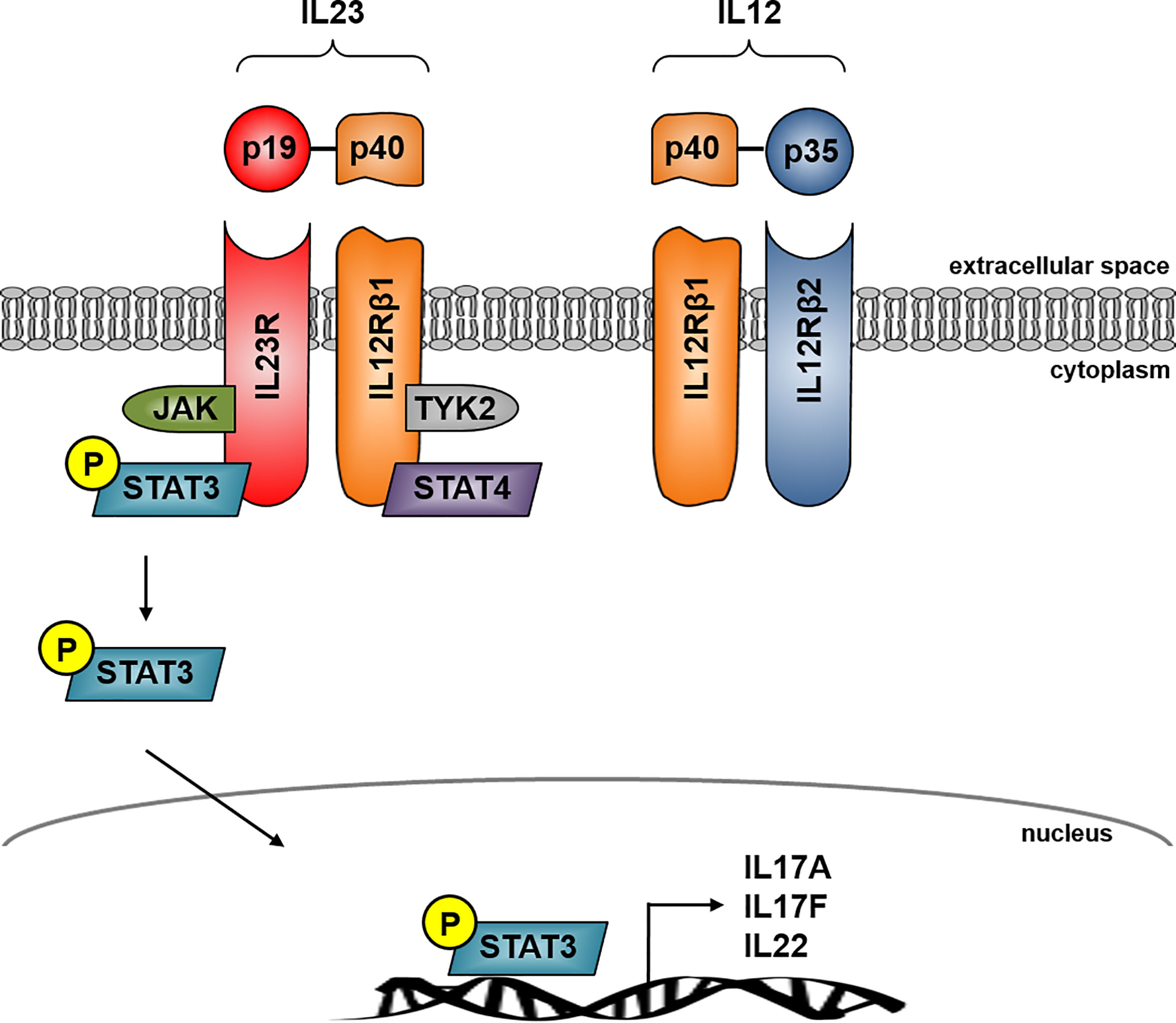



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology



Apilimod Inhibits The Production Of Il 12 And Il 23 And Reduces Dendritic Cell Infiltration In Psoriasis




Monoclonal Antibodies Inhibiting Il 12 23 And 17 For The Treatment Of Psoriasis Abstract Europe Pmc




Interleukin 12 Il 12 And Il 23 Signal Transduction Pathways Il 12 Download Scientific Diagram
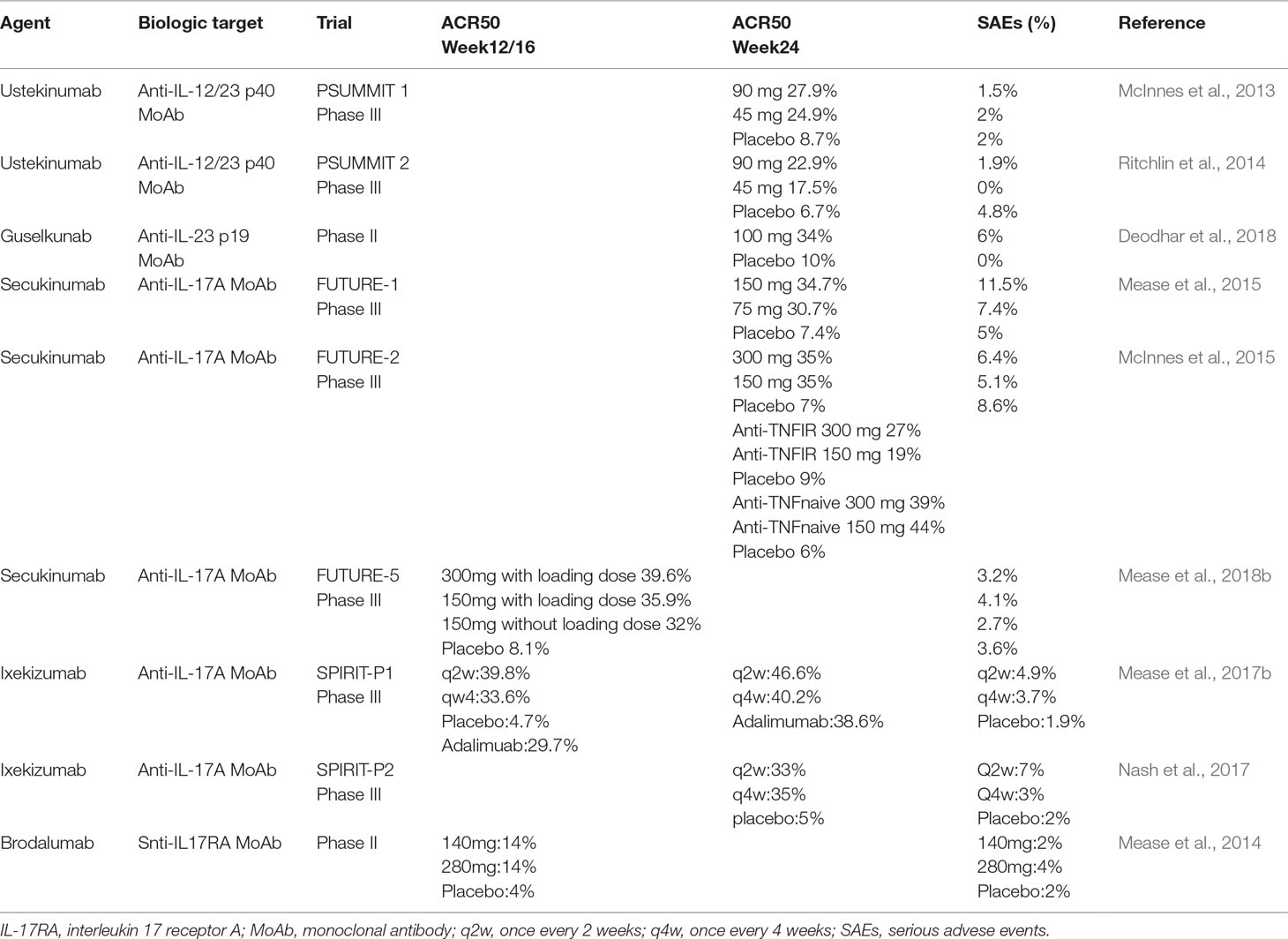



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology
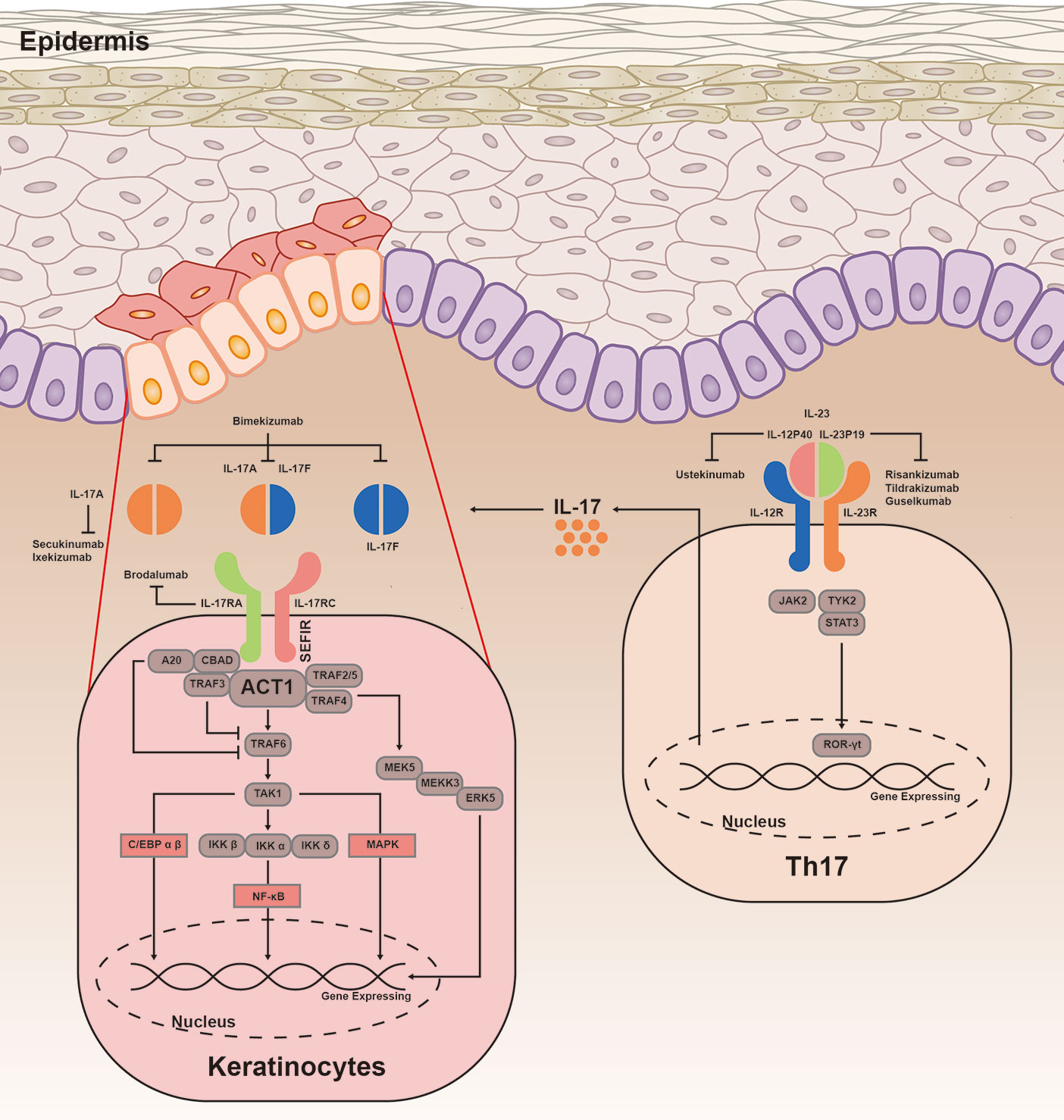



Frontiers The Il 23 Il 17 Pathway In Inflammatory Skin Diseases From Bench To Bedside Immunology




Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases




Figure 1 From Selective Interleukin 23 P19 Inhibition Another Game Changer In Psoriasis Focus On Risankizumab Semantic Scholar




Crohn S Disease Market Growth To 26 Fuelled By Interleukin Inhibitor And Anti Integrin Therapy Launches Globaldata




Efficacy And Safety Of Ustekinumab An Il 12 And Il 23 Inhibitor In Patients With Active Systemic Lupus Erythematosus Results Of A Multicentre Double Blind Phase 2 Randomised Controlled Study The Lancet




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology




Pdf Interleukin Il 12 And Il 23 And Their Conflicting Roles In Cancer




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt
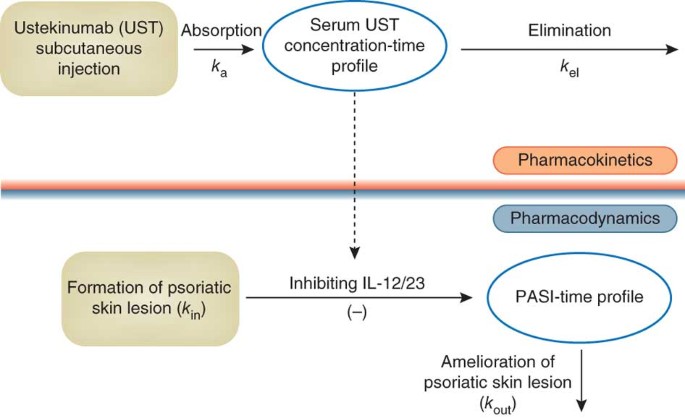



Therapeutic Targeting Of The Il 12 23 Pathways Generation And Characterization Of Ustekinumab Nature Biotechnology




Structural Basis For Il 12 And Il 23 Receptor Sharing Reveals A Gateway For Shaping Actions On T Versus Nk Cells Sciencedirect




Inhibition Of Interleukin 12 And Or Interleukin 23 For The Treatment Of Psoriasis What Is The Evidence For An Effect On Malignancy Ergen 18 Experimental Dermatology Wiley Online Library




Interleukin 23 Wikipedia
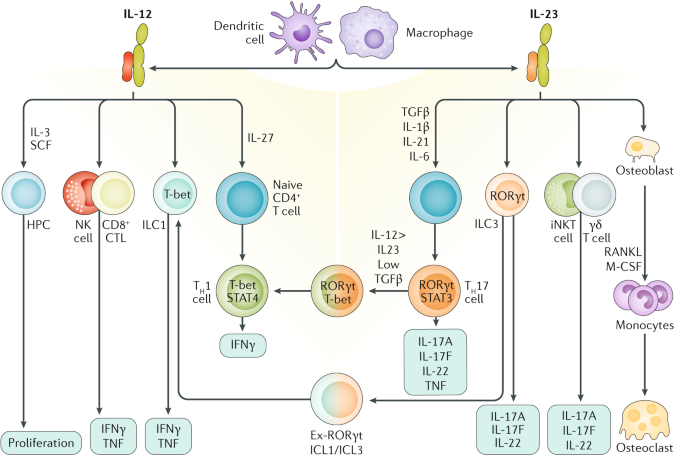



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Review Of Ustekinumab An Interleukin 12 And Interleukin 23 Inhibitor Used For The Treatment Of Plaque Psoriasis Abstract Europe Pmc
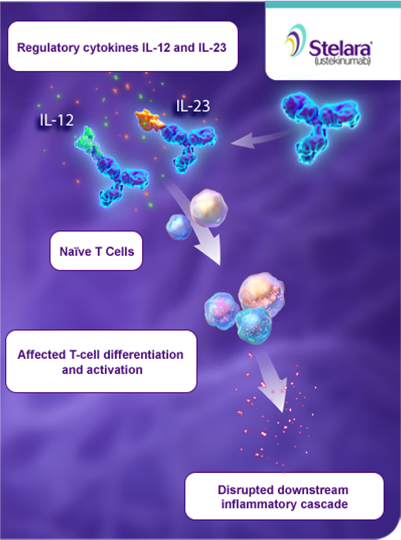



Stelara Ustekinumab Mechanism Of Action Plaque Psoriasis
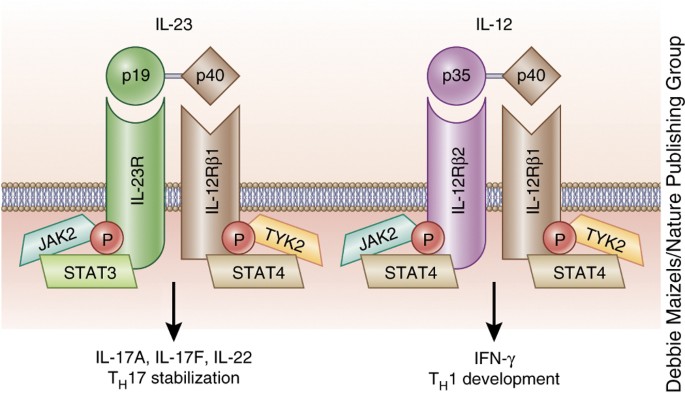



Il 12 And Il 23 Cytokines From Discovery To Targeted Therapies For Immune Mediated Inflammatory Diseases Nature Medicine




Structure Activity Relationship Study Target Identification And Pharmacological Characterization Of A Small Molecular Il 12 23 Inhibitor Apy01 Sciencedirect




Paradoxical Gastrointestinal Effects Of Interleukin 17 Blockers Annals Of The Rheumatic Diseases




New Biologic Therapies That Target The Il 12 23 Pathway Youtube




Scielo Brasil Biologic Therapy For Psoriasis Still Searching For The Best Target Biologic Therapy For Psoriasis Still Searching For The Best Target
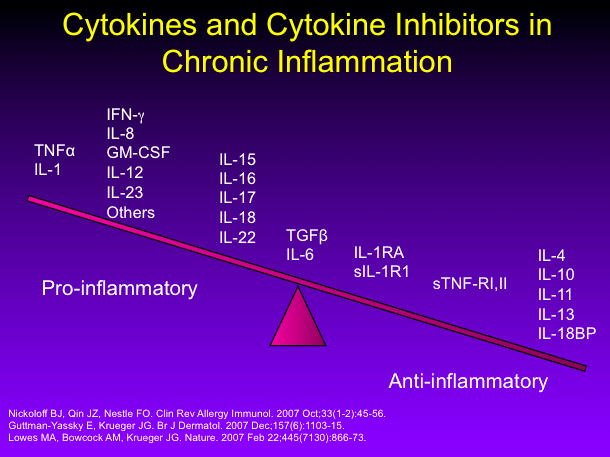



Psoriasis Archives Page 4 Of 6 Maui Derm




Management Of Plaque Psoriasis A Review And Comparison Of Il 23 Inhibitors European Medical Journal
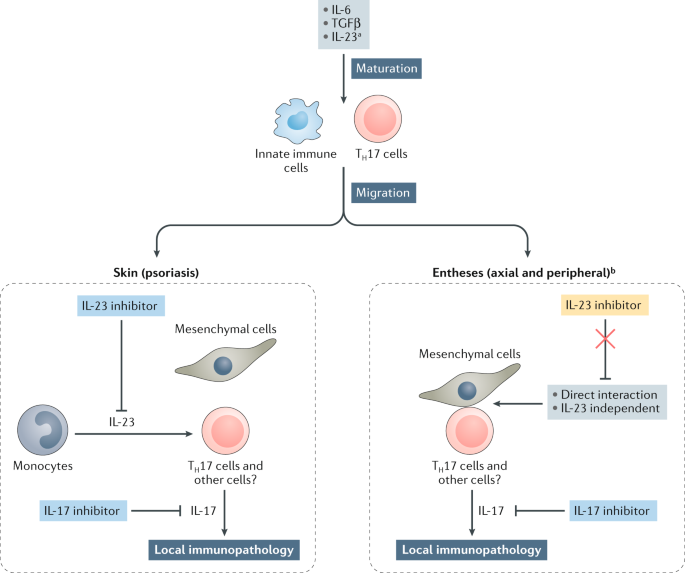



The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology




Psoriasis Translating Current And Emerging Therapies Into Enhanced Management Strategies Youtube




Interleukin Il 12 And Il 23 Structure And Receptors Notes The Il 12 Download Scientific Diagram




The Risk Of Respiratory Tract Infections And Interstitial Lung Disease With Interleukin 12 23 And Interleukin 23 Antagonists In Patients With Autoimmune Diseases A Systematic Review And Meta Analysis Journal Of The American




Pdf Review Of Ustekinumab An Interleukin 12 And Interleukin 23 Inhibitor Used For The Treatment Of Plaque Psoriasis




From Evolution To Revolution Il 23 In The Treatment Of Psoriasis Patients European Medical Journal




Spotlight On Risankizumab And Its Potential In The Treatment Of Plaque Ptt




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt



Arthritis News Ustekinumab An Inhibitor Of Il 12 23 Studied In Patients With Psoriatic Arthritis



0 件のコメント:
コメントを投稿